Indicator For Titration Strong Base
Weak acid strong base titration eg phenolphthalein thymolpthaline thymol blue Strong acid weak base titration eg Methyl orange methyl red bromophenol bromocresol green. Phenolphtalein is chosen because it changes color in a pH range between 83 10.
Which Indicator Is Used In A Strong Acid Versus A Strong Base Solution Quora
A drop of indicator solution is added to the titration at the beginning.
Indicator for titration strong base. A strong acid- strong base titration is performed using a phenolphthalein indicator. Instead it could be used for a strong acid-weak base titration where the pH at the equivalence point is. In an alkaline solution methyl orange is yellow and the structure is.
It will appear pink in basic solutions and clear in acidic solutions. A strong acid-strong base titration is performed using a phenolphthalein indicator. The endpoint has been reached when the color changes.
Hydrochloric acid a strong acid was used with sodium hydroxide a strong base. An aqueous solution of sodium hydroxide NaOH aq is a strong base. For a weak acid theres only partial ionization.
Below is the balanced chemical reaction for the reaction between CH 3. Now that weve looked at titration curves in great detail lets see how we could use an acid-base indicator to find the equivalence point for a titration so an indicator changes color and a specific pH range for example methyl red is an indicator that goes from red to yellow over a pH range of about four point four to six point two so we could say thats a pH range of approximately four to six bromothymol blue is an. In addition the anion negative ion created from the dissociation of the acid combines with the cation positive ion created from the dissociation of the base to create a salt.
Redox indicators are also used. It will appear pink in basic solutions and clear in acidic solutions. Indicators used in various titration Strong acid strong base titration eg methyl orange methyl red phenolphthalein bromothymol blue phenol red.
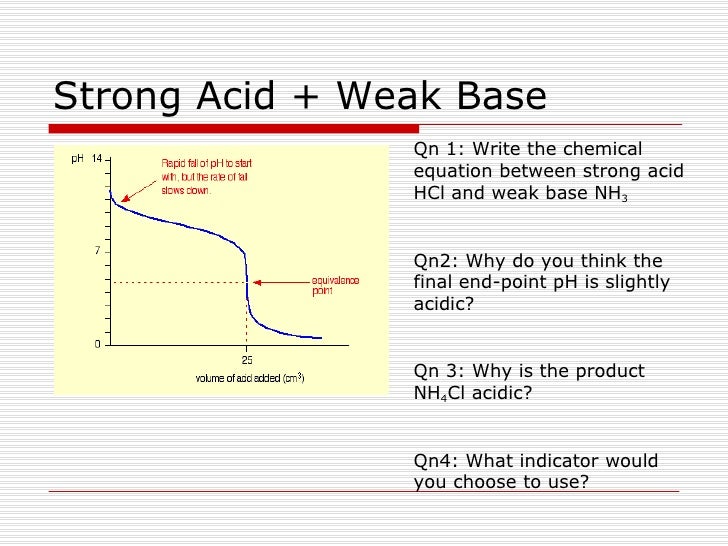
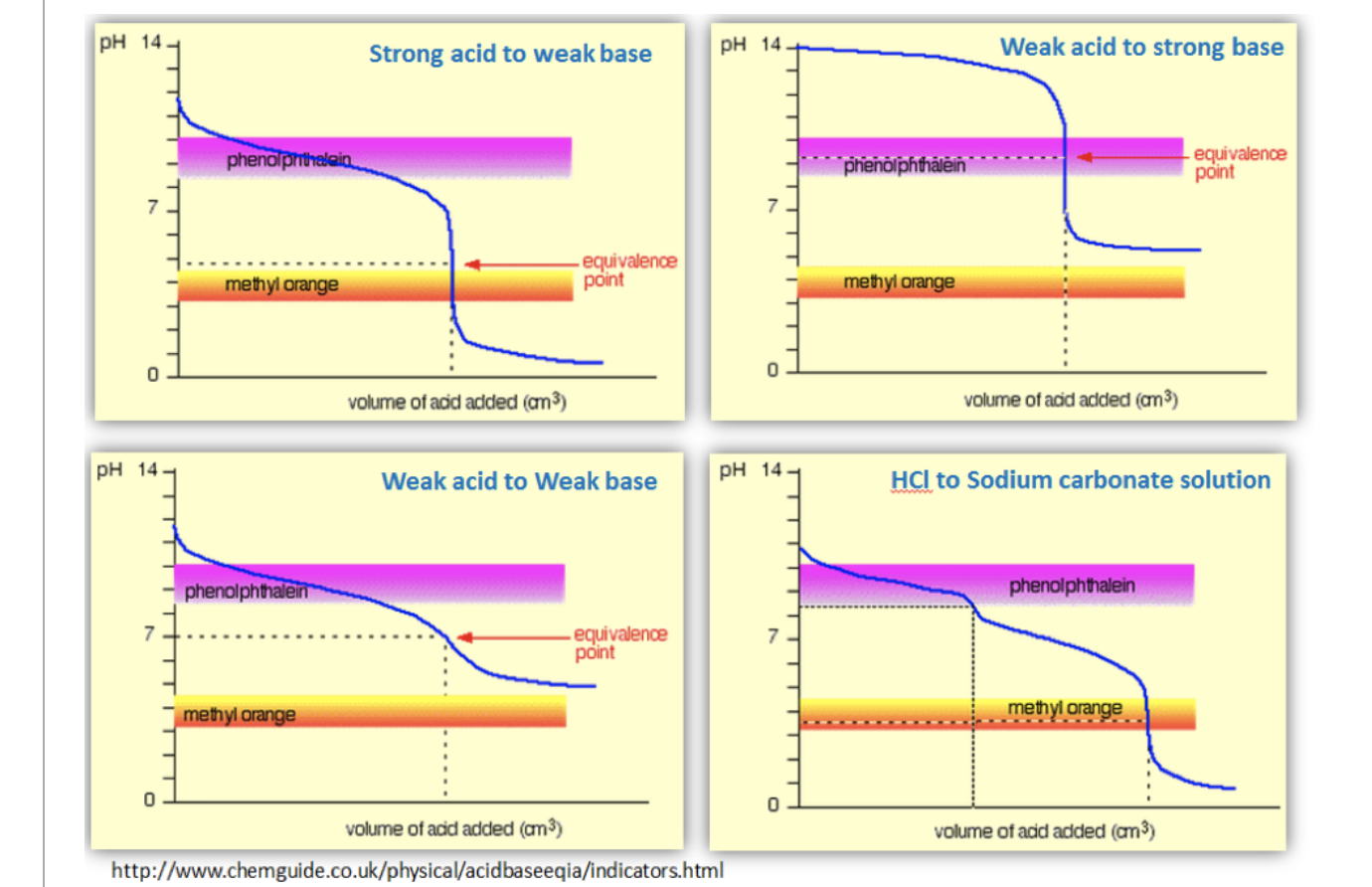
During the titration of strong acid with strong base the pH changes from 3 to 11 phenolphthalein indicator range from pH 8 to 10 thats why mostly used for this type of titration. Bromphenol blue would not be a good choice as the indicator for a strong acid-strong base titration because the pH is 7 at the equivalence point. Now you might think that when you add an acid the hydrogen ion would be picked up by the negatively charged oxygen.
An acidbase indicator eg phenolphthalein changes color depending on the pH. Phenolphtalein is chosen because it changes color in a pH range between 83 10. For the titration of a monoprotic strong acid HCl with a monobasic strong base NaOH we can calculate the volume of base needed to reach the equivalence point from the following relationship.
2 For titration of weak acid like acetic Acid against a strong base only phenolphthalein is a suitable indicator. 9 rows Thus in strong acid- strong base titrations any one of the above indicators can be used. Methyl orange is one of the indicators commonly used in titrations.
When a strong acid like HCl is titrated against a weak base like Na2CO3 the pH changes from 35 to 75 at the end point. The pH indicator used in this lab was phenolphthalein which is clear in acidic solutions and pink in basic solutions. Of excess base present.
Phenolphthalein indicator used in acid-base titration. 1 For titration of a strong acid against a strong base any indicator out of methyl orange methyl red phenolphthalein or bromothymol blue can be used to determine the endpoint. The best indicator for this type of titration is methyl orange which changes its colour within this pH range.
The pH at the equivalence point for this titration will always be 70 note that this is true only for titrations of strong acid with strong base. What factors govern the choice of a suitable indicator for an acid base titration. The equivalence point for the titration of a strong acid with a strong base occurs when OH exactly equals H 3 O in the solution.
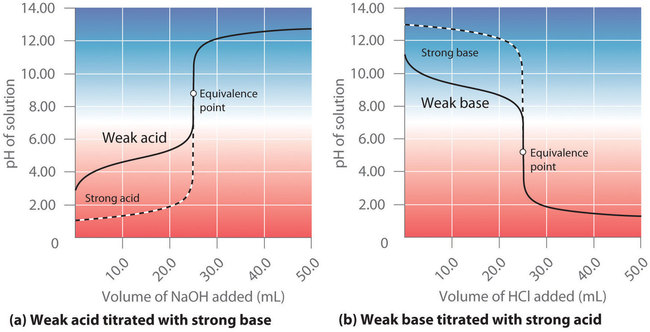
4Titration of weak acid against weak base. The situation in the case of the titration of a weak acid with a strong base is somewhat different due to the fact that a weak acid is only partially ionized in aqueous solution. 1 pH range of the indicator cuts the steepest part of the pH.
A drop of indicator is added in the start of the titration the. Strong acids and strong bases completely ionize in solution resulting in water and a salt. A substance that changes color of the solution in response to a chemical change.
Choosing an Appropriate Indicator for a Weak Acid - Strong Base Titration An aqueous solution of acetic acid ethanoic acid CH 3 COOH aq is a weak acid. Moles of base volumebmolaritybVbMb moles of acid volumeamolaritya VaMa.
Acid Base Titration Curves Artykul Khan Academy
Chm 2046c Module 12 Part D Acid Base Titration Curves
Ph Understanding Titration Curve
7 3 Acid Base Titrations Chemistry Libretexts
Solved 1 Figure 1 Shows The Titration Curves Of Four Aci Chegg Com
Learn Titration Curve Meaning Concepts Formulas Through Study Material Notes Embibe Com
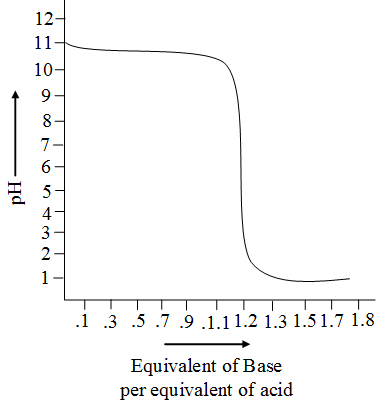
What Indicator Is Used In Strong Acid And Weak Base Titration And Why Quora
Titration Curve Weak Base Strong Acid Ppt Download

General Chemistry Online Faq Acids And Bases How Can Strong And Weak Acids Be Distinguished Using Indicators
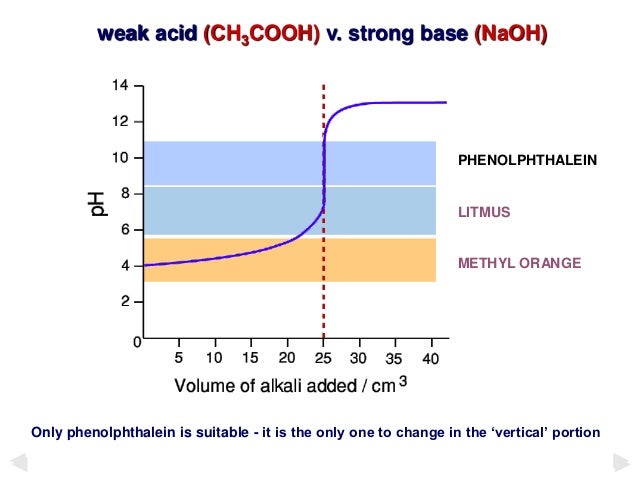
Theory Of Acid Base Indicators And Acid Base Titration Curves

Acid Base Titration Indicators Titration A Method Of

Titration Chemistry For Non Majors

Strong Acid Weak Base Titrations Introduction To Chemistry

Weak Acid Strong Base Titrations Introduction To Chemistry

Why Is Phenolphthalein An Appropriate Indicator For Titration Of A Strong Acid With A Strong Base Chemistry Stack Exchange
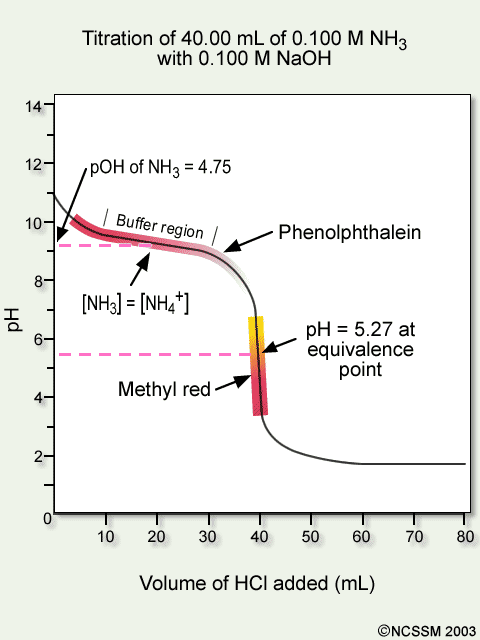
Tiger Ncssm Distance Education And Extended Programs
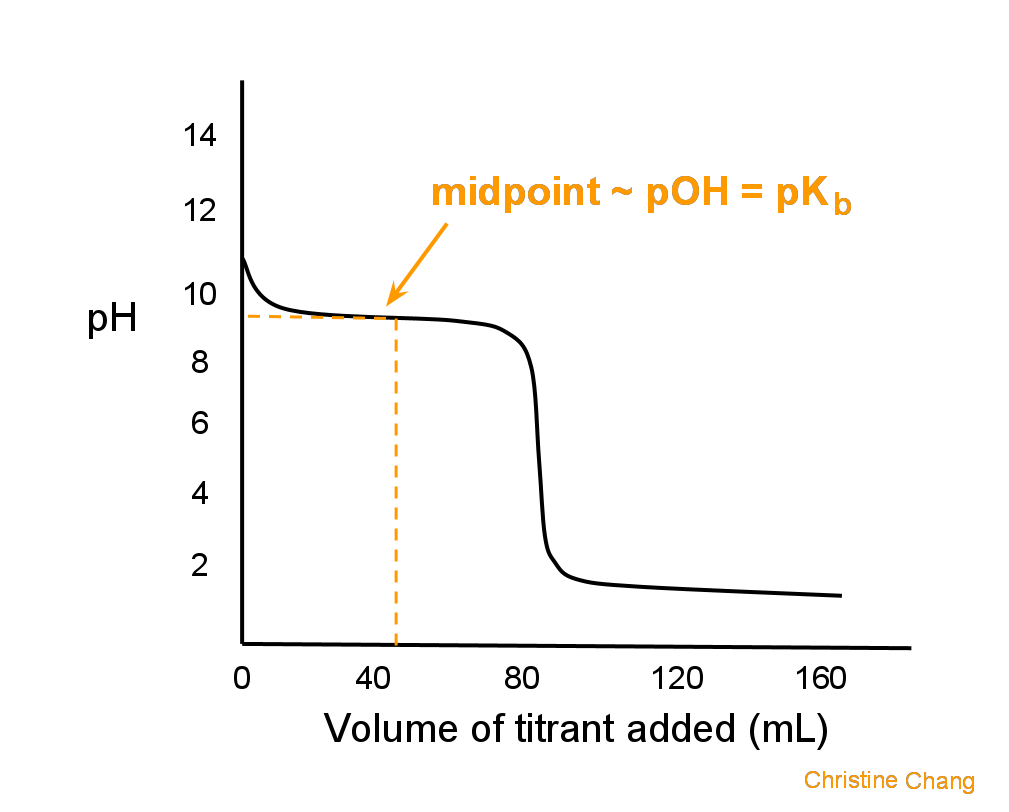
Titration Of A Weak Base With A Strong Acid Chemistry Libretexts

Acid Base Titration Titration Curves Equivalence Point Indicators With Videos

Post a Comment for "Indicator For Titration Strong Base"