What Is The Difference Between Volumetric Titration And Potentiometric Titration
A volumetric analysis can be used to determine the concentration of a solution the molar mass of a component in a solution the percentage of a component present in a solution formula of a substance or stoichiometry of an. Potentiometric titrations operate on the same general principle except that an electrode is inserted into the analyte solution and connected to a volt meter.
The potential voltage of the analyte is then monitored as titrant is added.

What is the difference between volumetric titration and potentiometric titration. The most straight forward and mostly used method of end point detection in potentiometric titration is plotting a graph between. The key difference between potentiometric and conductometric titrations is that potentiometric titrations measure the potential across the analyte whereas conductometric titrations measure the electrolytic conductivity of the analyte. Titrations can be based on redox reactions.
Moreover volumetric titrations are easy and quick when compared to potentiometric titrations. One Component Reagent Less expensive and easier to handle but has a less stable titer and slower titration speed. Volumetric titrators are usually designed for moisture levels ranging from 100 ppm to 100 whereas coulometric titrators measure water content in the in potentiometric titration the titrator measures the potential between two electrodes.
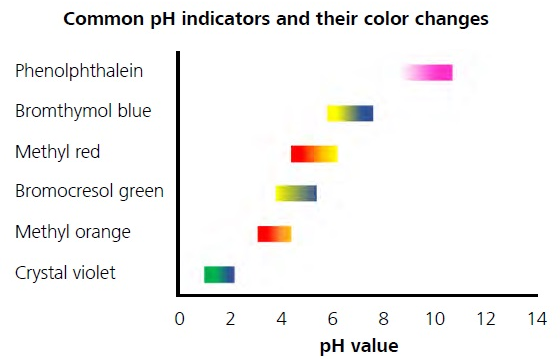
The indicators used in volumetric titrations undergo color change in a range of pH. Titrations can be classified as acid-base titration redox titration precipitation titration and complexometric titration. Potentiometric titration involves measurement of the potential of an indicator electrode with respect to a reference electrode as a function of titrant volume.
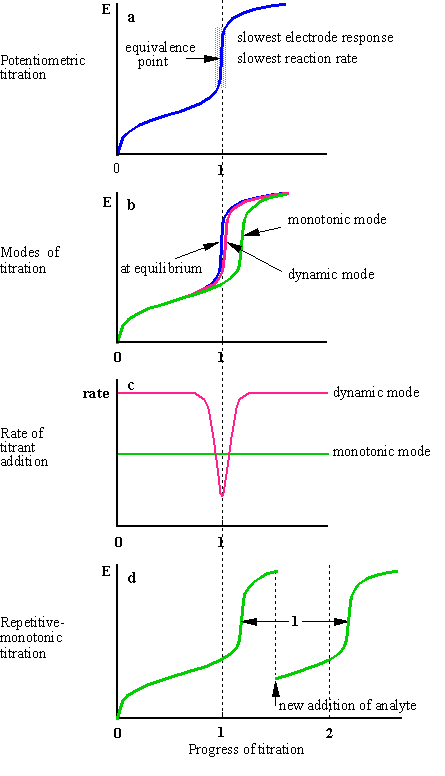
Potentiometric titrations involve the measurement of the potential difference between two electrodes of a suitable cell. -Potentiometric titrations are similar but the endpoint of the titration is found with potentiometry measuring of potentials in a chemical redox reaction. Endpoint is the point in the titration where the indicator changes its color.
After the titration process the potential difference between the two electrodes namely the reference and indicator electrode is measured in conditions where a thermodynamic equilibrium is maintained and the current passing through the electrodes does not disturb this equilibrium. Meaning electrons are being transferred between distinct chemical species in that reaction. -Potentiometric titrations are similar but the endpoint of the titration is found with potentiometry measuring of potentials in a chemical redox reaction.
Instead the potential is measured across the analyte typically an electrolyte solutionTo do this two electrodes are used an indicator electrode the glass electrode and metal ion indicator electrode and a reference electrode. And amperometric titrations the electric current passing during the course of the titration. Often volumetric analysis is also called titrimetric analysis or titration.
But there is a difference between volumetric analysis and titration when considering their applications. The key difference between volumetric and potentiometric titration is that volumetric titration measures the volume of analyte reacted with the reagent whereas potentiometric titration measures the potential across the analyte. No indicator is used.
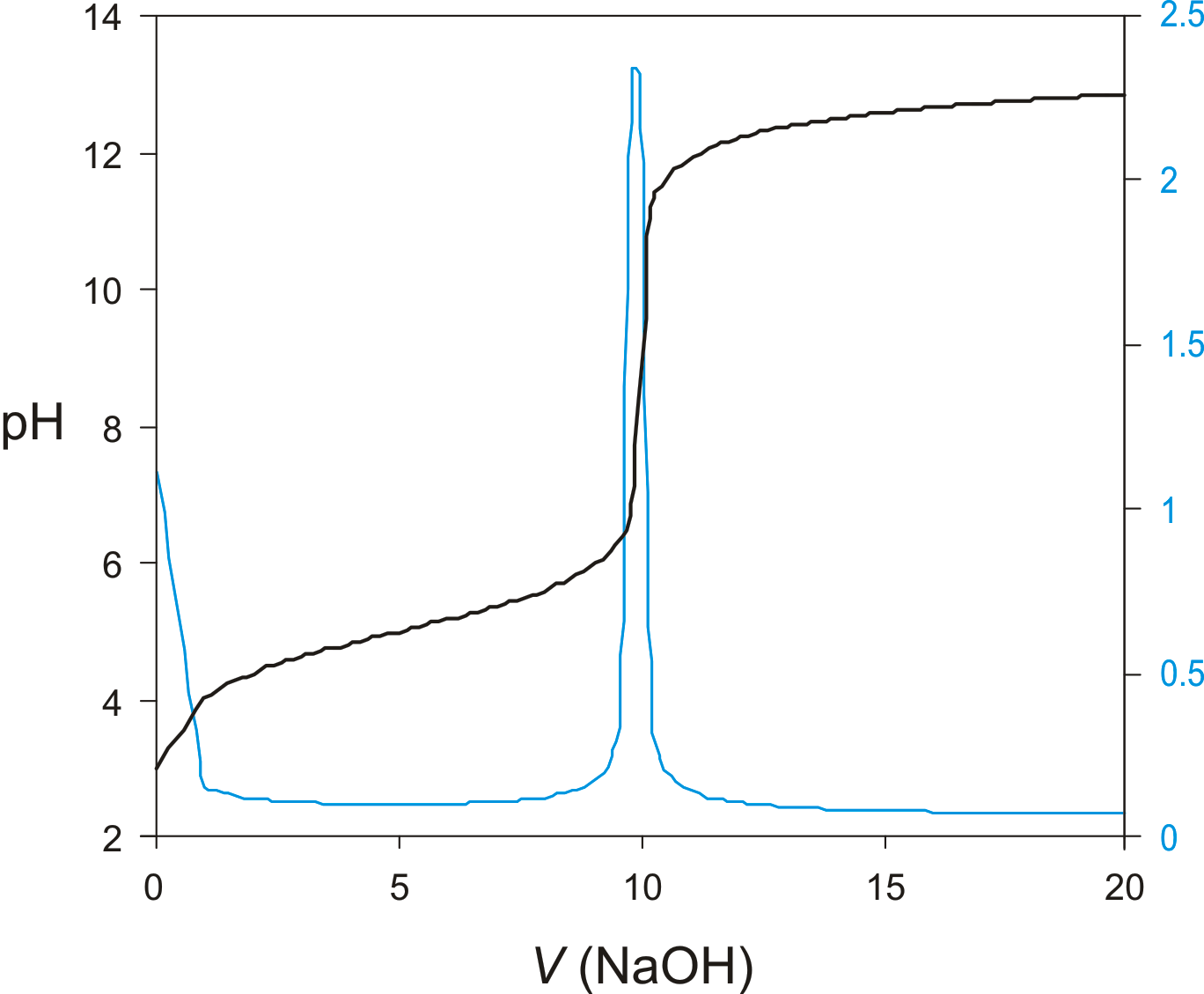
For example which neither a potentiometric nor indicator method can be used for the. It is a useful means of characterizing an acid. An equivalence point refers to the point in the titration where the volume of base added is equal to the volume of acid in the sample.
Titration is an analytical chemistry technique used to find the concentration of an unknown solution using a solution whose concentration is known. The chemist normally determines the end point later by constructing a graph of potential vs. This only applies to reactions where something is being oxidized and something is being reduced.
Meaning electrons are being transferred between distinct chemical species in that reaction. A titration is an analytical technique in which we can determine the concentration of an analyte. This only applies to reactions where something is being oxidized and something is being reduced.
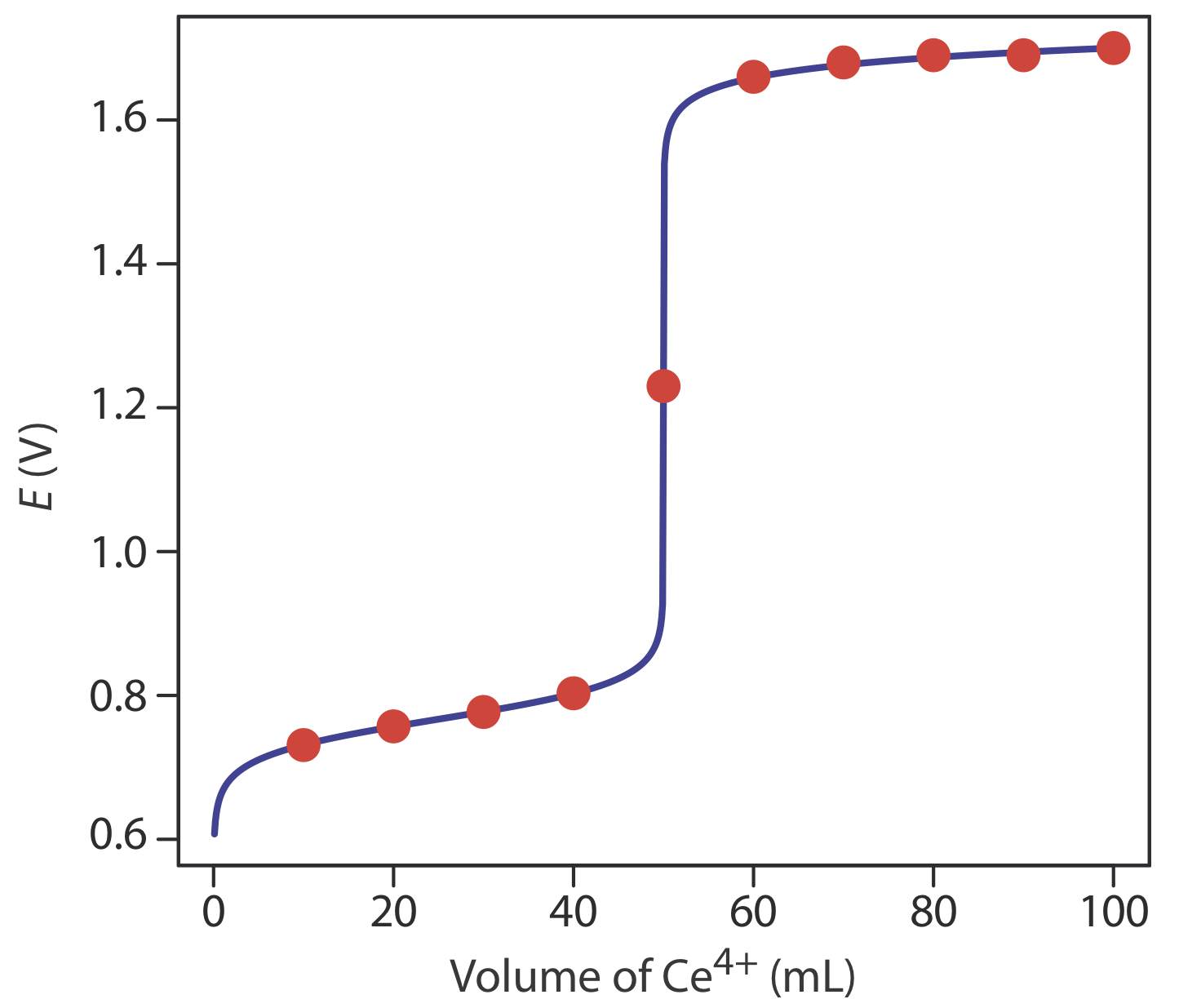
As we approach the end point we start adding titrants in very small quantities. Potentiometric titration is a volumetric method which is parallel to direct titration of a redox reaction. The objective here is to do a simple titration and use the titration curve to deduce the concentration and standard potentials of the redox couplesa potentiometric titration.
There are two types of volumetric KF systems. Potentiometric titration is a technique similar to direct titration of a redox reaction. The reaction considered is.
There is no difference between an equivalence point and an endpoint. In this titration we measure and record the cell potential in millivolts or pH after adding titrant each time. Conductometric titrations the electrical conductance or resistance of the solution being titrated.
June 18 2019 Posted by Madhu. Potentiometric titration uses an indicator electrode instead of an indicator to monitor the endpoint of the titration. This method is a useful way of characterizing an acid.
Two Component Reagent Has a longer term stability and faster titration time but have solvent capacity restrictions. The key difference between volumetric analysis and titration is that the term volumetric analysis is used where analysis is done to analyse a solution for several different unknown values whereas the term titration. However the end point in conductometric titration is sharp as direct change in conductivity can be obtained.
These terms can be used interchangeably. Karl Fischer Volumetric Titrator. Conductometric titrations have the following advantages over volumetric titration.
The main advantages to the conductometric titration are its applicability to very dilute and colored solutions and to a system that involves relative incomplete reactions.
Automated Vs Manual Titration Which Should You Use

What Is Potentiometric Titration Its Principle Method Types

Potentiometric Titration An Overview Sciencedirect Topics

Difference Between Potentiometric And Conductometric Titrations Compare The Difference Between Similar Terms

Difference Between Volumetric And Potentiometric Titration Compare The Difference Between Similar Terms

Titration Definition Types Facts Britannica
Chemistry Glossary Search Results For Potentiometric Titration

Difference Between Volumetric And Potentiometric Titration Compare The Difference Between Similar Terms

Scielo Brasil Potentiometric Titration And Out Of Equilibrium Ph Response Of The Biotite Water System Potentiometric Titration And Out Of Equilibrium Ph Response Of The Biotite Water System

Potentiometric Titration An Overview Sciencedirect Topics

Difference Between Volumetric And Potentiometric Titration Compare The Difference Between Similar Terms

Kimya Chemistry Himiya Volumetric Titration Coulometric Titration Chemistry Analysis Generation

9 2 Acid Base Titrations Chemistry Libretexts
9 4 Redox Titrations Chemistry Libretexts


Post a Comment for "What Is The Difference Between Volumetric Titration And Potentiometric Titration"