Why No Indicator Needed For Redox Titration
That answer is simpler than many think it is because of the redox reaction that ll take place the KMnO4 plays both reactant that oxidizes the other and get it self reduced and indicator since itll looses its nice purple color when the reaction is over. Potassium permanganate can used as a self indicator in redox titrations where applicable.
The endpoint can be detected with a voltmeter.

Why no indicator needed for redox titration. In other redox reactions you might be measuring the change using an electrochemical signal like in voltammetric or conductometric titrations. KMnO4 has a very deep purple color and is self-indicating because the endpoint is. We can also determine by using a voltmeter to show the electric potential.
Some redox reactions involve transition metals which are usually colored and so the colors of the metal ions in solution will do the indicating. An indicator or potentiometer is used to find out the end point of titration main constituent in the oxidizing agent is potassium dichromate. In this experiment It is not essential to use an indicator.
Why is the tit. When added to a solution containing the ana- lyte the indicator imparts a color that depends on the solutions electrochemical potential. Thats just like using a pH meter for a pH titration.
But other redox reactions dont involve transition metals and these require an indicator. Discuss theory of redox titrations and indicators used. In the case of permanganate titrations you dont need a separate indicator because the permanganate ion itself has a distinctive easily detected purple color that can be detected.
Sometimes an indicator is required but many redox titrations have color changes that occur naturally due to the transfer of electrons. Non redox indicator change color when excess amount of titrant exists eg. 2 Explain why no indicator is needed in this experiment.
Some redox titrations do not require an indicator due to the intense color of the constituents. My question is what role other than a dehydrating agent and maybe catalysing does the ceH2SO4 play in this titration. 7 rows The most important class of indicators are substances that do not participate in the redox.
Pharmaceutical Analysis - I Shom Prakash Kushwaha Hygia redox indicator redox titr. The titration is between oxalic acid and potassium permanganate with ceH2SO4 added to oxalic acid. Because the endpoints are easily observed.
You dont strictly need. Starch changes to deep blue color when excess amount I. When used in redox titration it get reduced into brown coloured Mn2 ion In acidic media at end point and colour.
Redox reactions are carried out in the same way as acid-base titrations using a burette and a known concentration of one reactant titrant and an unknown concentration of the other reactant analyte. For instance when iodine is formed as I3- in a redox reaction starch is used as an indicator because iodine and starch form a dark blue complex. For an example in the first part color of.
This is because without a pH change the endpoint of the reaction can be determined using only by the colour change. Since detecting end point is the role of indicator later is not required in Permanganate titration also. But some titration do not need an indicator.
Why is an indicator not used in a redox titration. The most important class of visual indicators however are substances that do not participate in the redox titration but whose oxidized and reduced forms differ in color. Indicator electrode Redox indicators the indicator has different color at reduction and oxidation state.
There were no indicators needed in the experiment because the chemicals themselves that are being oxidized or reduced serve as indicators because redox involves completely changing the electronic states of the element that is often colored. Redox titrations use potassium permanaganate KMnO4 as titrant against a solutionanalyte containing Fe2 ions. The color changes is not definite thats why sodium diphenylamine is used.
Potassium Permanganate is an oxidizing agent which is of deep violet colour. Of KMnO4 with sodium oxalate done without indicator Or something like that. This is because its reduction product Cr3 is green which hinders in the visual detection of end point by observing dichromate colour.
Explain why no indicator is needed in this experiment. Thus an indicator is must in this titration. For instance in permanganometry a slight persisting pink color signals the endpoint of the titration because of the color of the excess oxidizing agent potassium permanganate.
When used in redox titration it get reduced into brown coloured Mn2 ionIn acidic media at end point and colour change at end point can be detected easily.

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download

9 4 Redox Titrations Chemistry Libretexts

9 4 Redox Titrations Chemistry Libretexts
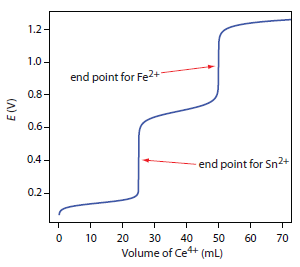
9 4 Redox Titrations Chemistry Libretexts
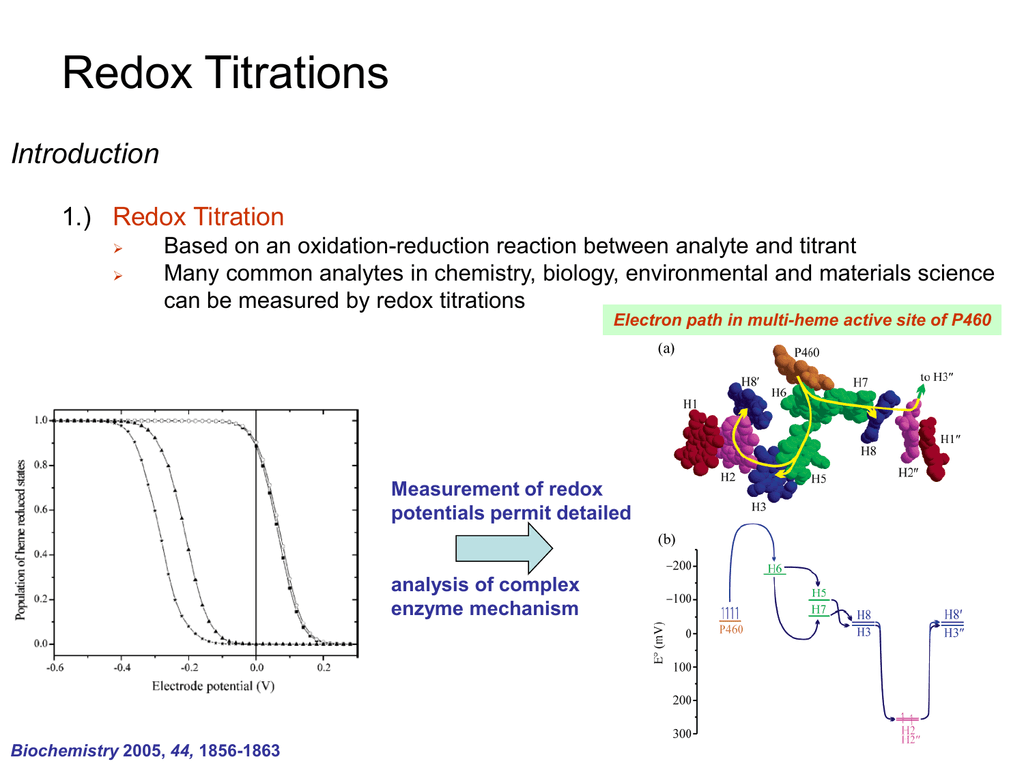
Redox Titrations Introduction 1 Redox Titration

Notes On Redox Titration Notes

Redox Titrations Introduction 1 Redox Titration Ppt Video Online Download
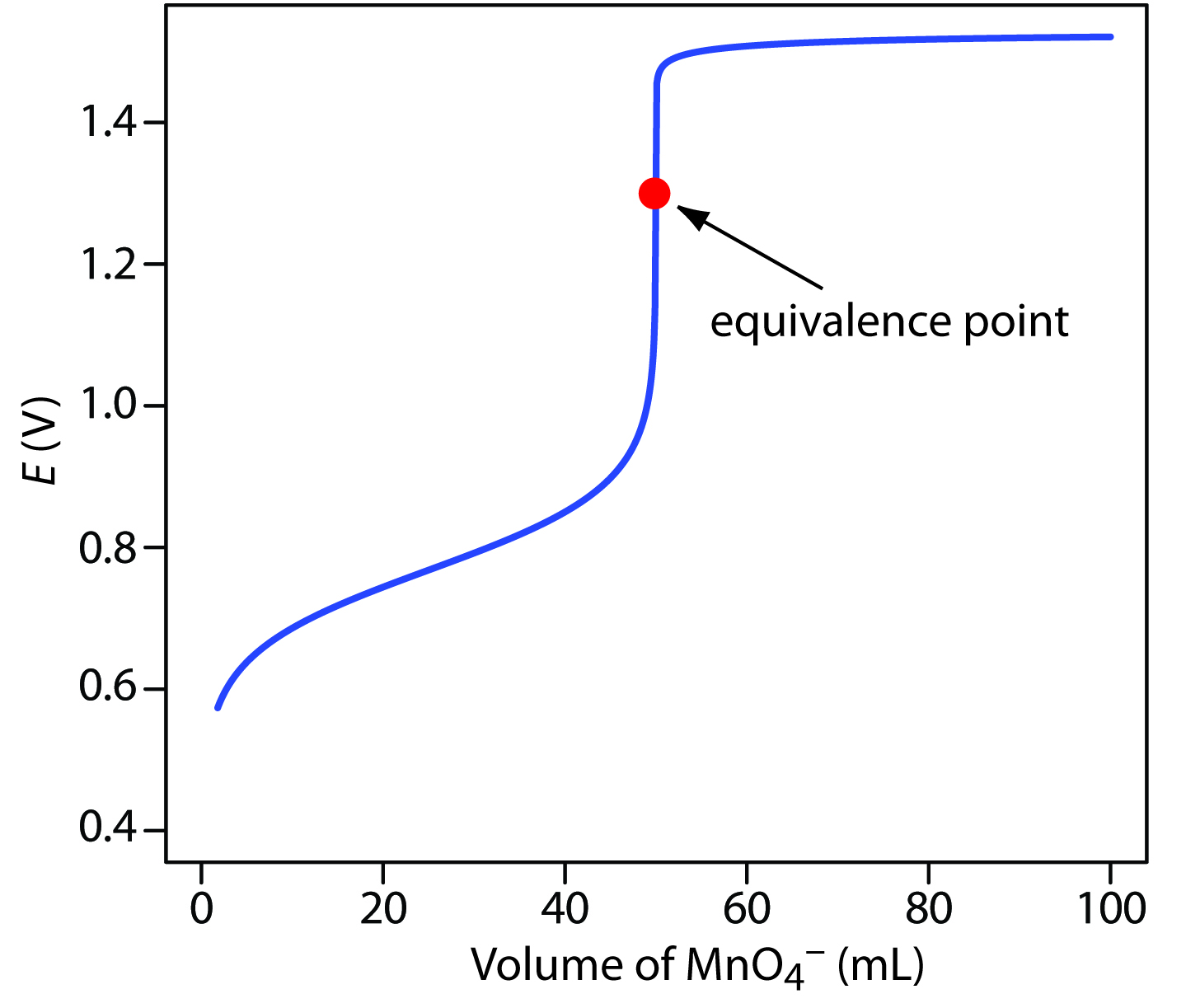
9 4 Redox Titrations Chemistry Libretexts

A Level Gce Quantitative Analysis Redox Volumetric Titration Calculations Potassium Manganate Vii Permanganate Iodine Sodium Thiosulphate Thiosulfate Potassium Dichromate Vi Ethanedioate Ion Oxalates Ethanedioic Acid Oxalic Acid Exam Practice
Difference Between Acid Base Titration And Redox Titration
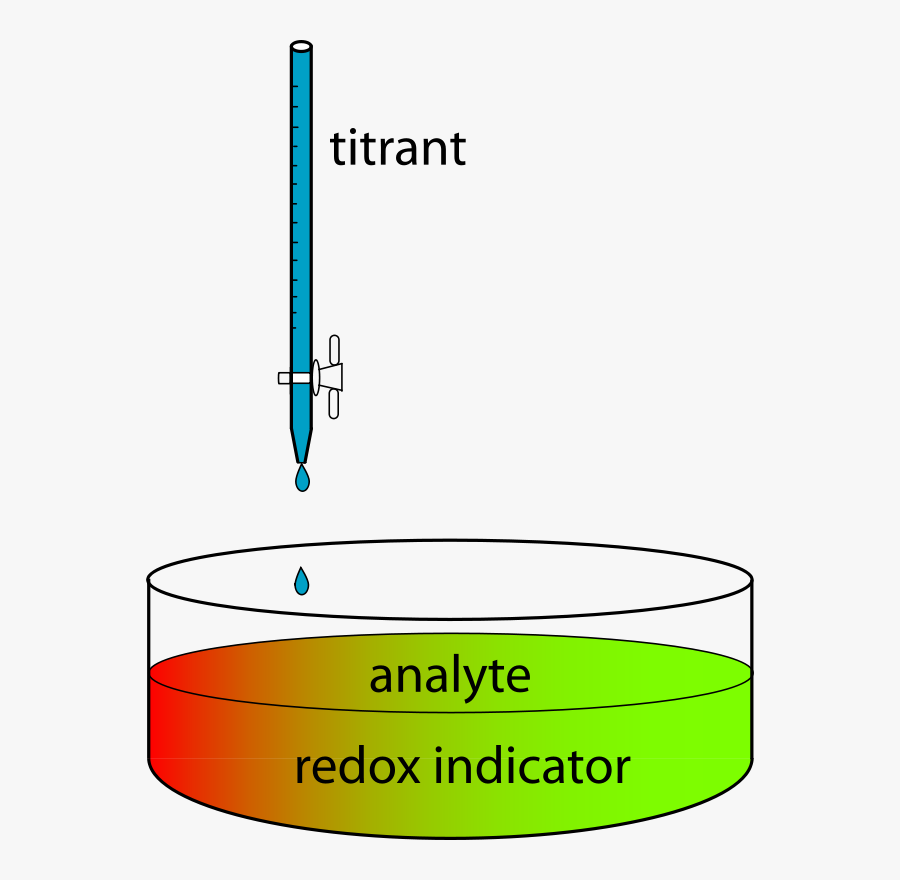
Free Clip Art Redox Titration Using Indicator Oxidation Reduction Titration Diagram Free Transparent Clipart Clipartkey
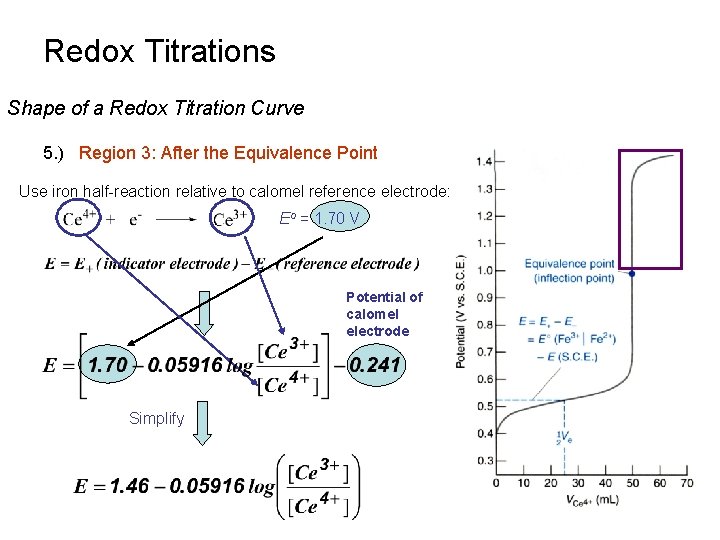
Redox Titrations Introduction 1 Redox Titration Based On

Redox Titrations A Redox Titration Is The Same As An Acid Base Titration Except It Involves A Redox Reaction And Generally Does Not Require An Indicator Ppt Download
Selecting An Indicator For A Redox Titration Image And Video Exchange Forum

Q 21 Discuss Theory Of Redox Titrations And Redox Indicators By S P Kushwaha Hiper India Youtube


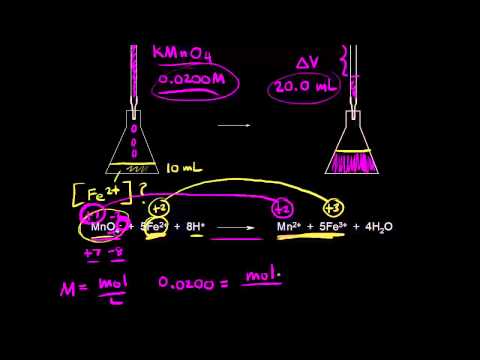
Post a Comment for "Why No Indicator Needed For Redox Titration"