With The Use Of Universal Indicator Detect The Acid Base And Salt From The Given Solution
Given the salt predict that acid-base pair that would produce that salt. They are cheap rapidly produce a result that is good to about a pH unit and can simply be discarded after use.
Identify Salts As Neutral Acidic Or Basic Video Khan Academy
The pH scale ranges between 1 and 14.
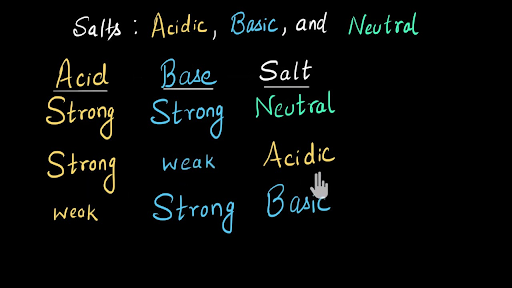
With the use of universal indicator detect the acid base and salt from the given solution. You can check the reaction mixture using universal. 2 Bases turn red litmus to blue. Universal Indicator which is a solution of a mixture of indicators is able to also provide a full range of colors for the pH scale.
Litmus is the most common indicator for testing acids and bases in the laboratory. PH is defined as the negative logarithm base 10 of the hydrogen ion concentration in moles per litre. At the endpoint has been reached the colour changes.
Universal Indicator Methyl Red Thymol Blue and Cabbage Juice. A drop of the solution to be tested is put on a strip of universal indicator paperIt then undergoes a colour. Heat the solution to evaporate the liquid and obtain the salt Acid base -- salt water Sulphuric acid iron II oxide -- iron sulphate water H2SO4 FeO -- FeSO4 -- H2O.
An acid-base indicator is a substance that changes its colour depending on the pH of the solution that it is added to. A pH indicator is a substance that it changes its colour in response to a chemical change. Redox indicators are also frequently used.
Universal indicators are good to about a pH unit. A properly selected acid-base indicator can be used to visually indicate the approximate pH of a sample. Acids are substances that generate free hydrogen ions H ions when dissolved in water.
When a solution is added to a cloth strip treated with onion extract then the smell of onion cannot be detected. Well first the advantages. Universal indicator is a mixture of many indicators which gives diferent colours at different pH values of entire scale.
Unlike litmus universal indicator can show us how strongly acidic or. It is a mixture of several different indicators. Universal indicator is a type of pH indicator that gives its color changes for a wide variety of pH values ranging from 0 to 14.
NEUTRALISATION usually involves mixing an acid pH 7 which react to form a neutral SALT solution of pH 7 in general the word equation for a neutralisation reaction is ACID BASEALKALI SALT WATER. 02 Acid bases and salt An indicator is a dye that shows a change in colour when brought in contact with acids and bases. Bases have pH values higher than 7.
A If a drop of the given solution turns blue litmus paper to red then the given solution will be acidic in nature or it will be an acid. Universal indicator is supplied as a solution or as universal indicator paper. Acids have pH values lower than 7.
PH -log 10 H Chemicals Required. Difference Between Acid Base Indicator and Universal Indicator Definition. It shows different colours at different concentration of ions in the solution.
A value less than 7 on the pH scale represents an acidic solution whereas basic solution has pH value more than 7. Alkalis give bluepurpleviolet colour with universal indicator or litmus paper. To measure the strength of an acid or a base solution we use universal indicators.
So the disadvantage has to do the the latter two points. This tells us whether the substance we are testing is an acid or base with respect to the change of colour. One of the ways pH can be measured is with an acid-base indicator such as universal indicator.
A variety of indicators change color at various pH levels. An acid-base indicator changes its colour depending on the pH eg phenolphthalein. The given solution contains base because onion extract loses its smell when added to a base but its smell does not change when added to acid.
Bases are substances when dissolved in water produces hydroxyl ions OH- ions. State whether the given solution contains an acid or base. 1 Acids turn blue litmus to red.
It can be difficult to add exactly the right amount of acid and alkali in a neutralisation reaction so that your salt solution is precisely pH 7. Acid base indicators are chemical substances that can give a color change in a reaction medium as a response to a change in pH. A drop of indicator solution is added to the titration at the start.
Determination Of pH Of Some Solutions Obtained From Fruit Juices Solution Of Known And Varied Concentrations Of Acids Bases And Salts Using pH Paper Or Universal Indicator. A reaction in which an acid and a base react in an aqueous solution to produce a salt and water.

Indicators Acids Bases And The Ph Value Siyavula

Unit 8 Acids And Bases Part 1 Sl Material Only Part 2 Hl Material Only Ppt Download

Agf Chemistry Pot Acids Bases Salts

Acids Bases And Salts Bases Acids Salts Acids

Acids Bases And Salts Bases Acids Salts Acids
How To Differentiate Between Basic And Acidic Salt Quora

Acids Bases Salts Acids Bases And Salts The Word Acids Comes From One Of Its Characteristic Properties Its Taste Acids Acidus Latin Word Meaning Ppt Download
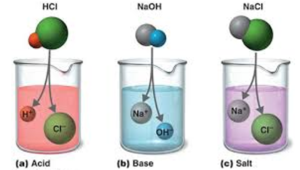
Acids Bases And Salts Experimental Definitions Arrhenius Concept Q A
How To Differentiate Between Basic And Acidic Salt Quora

Ncert Exemplar Class 10 Science Solutions Chapter 2 Download Pdf For Free

How Can We Distinguish Between An Acid And A Base Without The Use Of An Indicator Quora
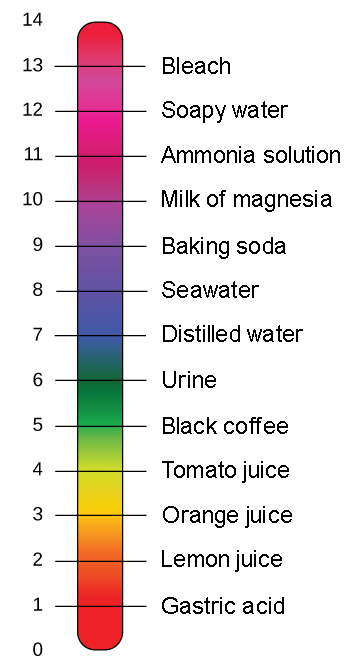
Buffers Ph Acids And Bases Biology For Non Majors I

Ph Paper Test On Acids Bases Salts Grade 10 Board Practical Chemistry Demonstration Youtube
How To Differentiate Between Basic And Acidic Salt Quora

Acid Base Properties Of Salts Boundless Chemistry


Post a Comment for "With The Use Of Universal Indicator Detect The Acid Base And Salt From The Given Solution"